SENARAI OYK/OYT STAF UTHM
SENARAI OYK/OYT STAF UTHM
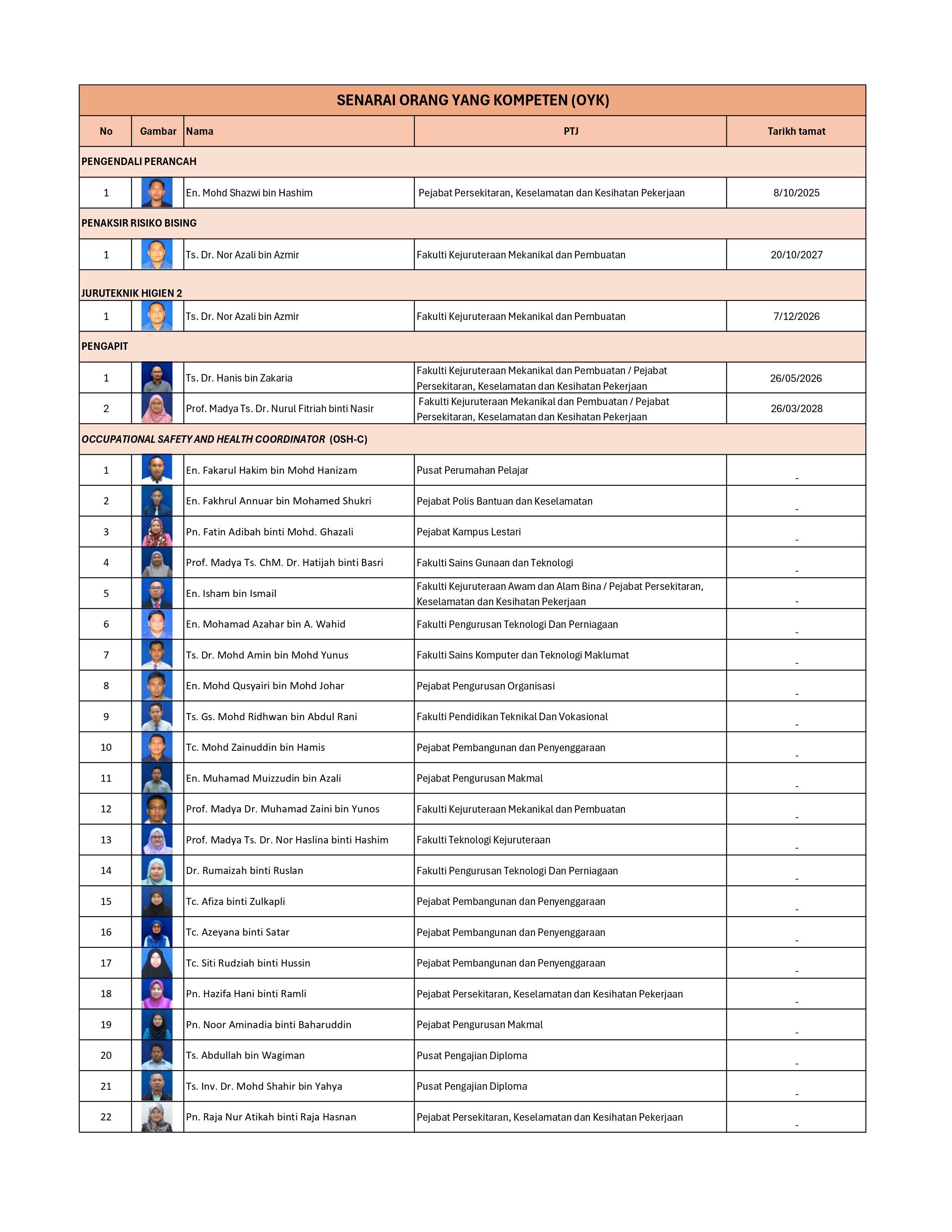
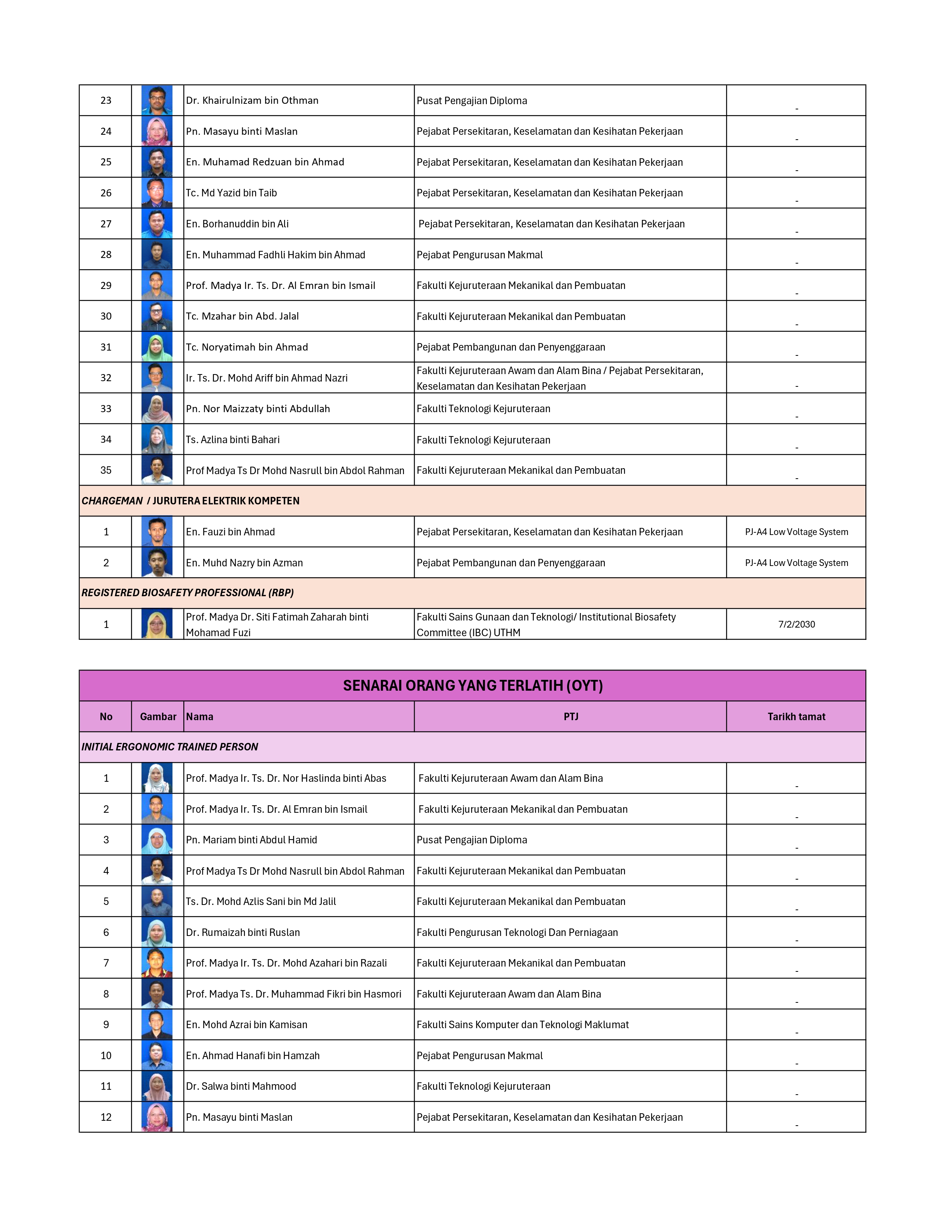
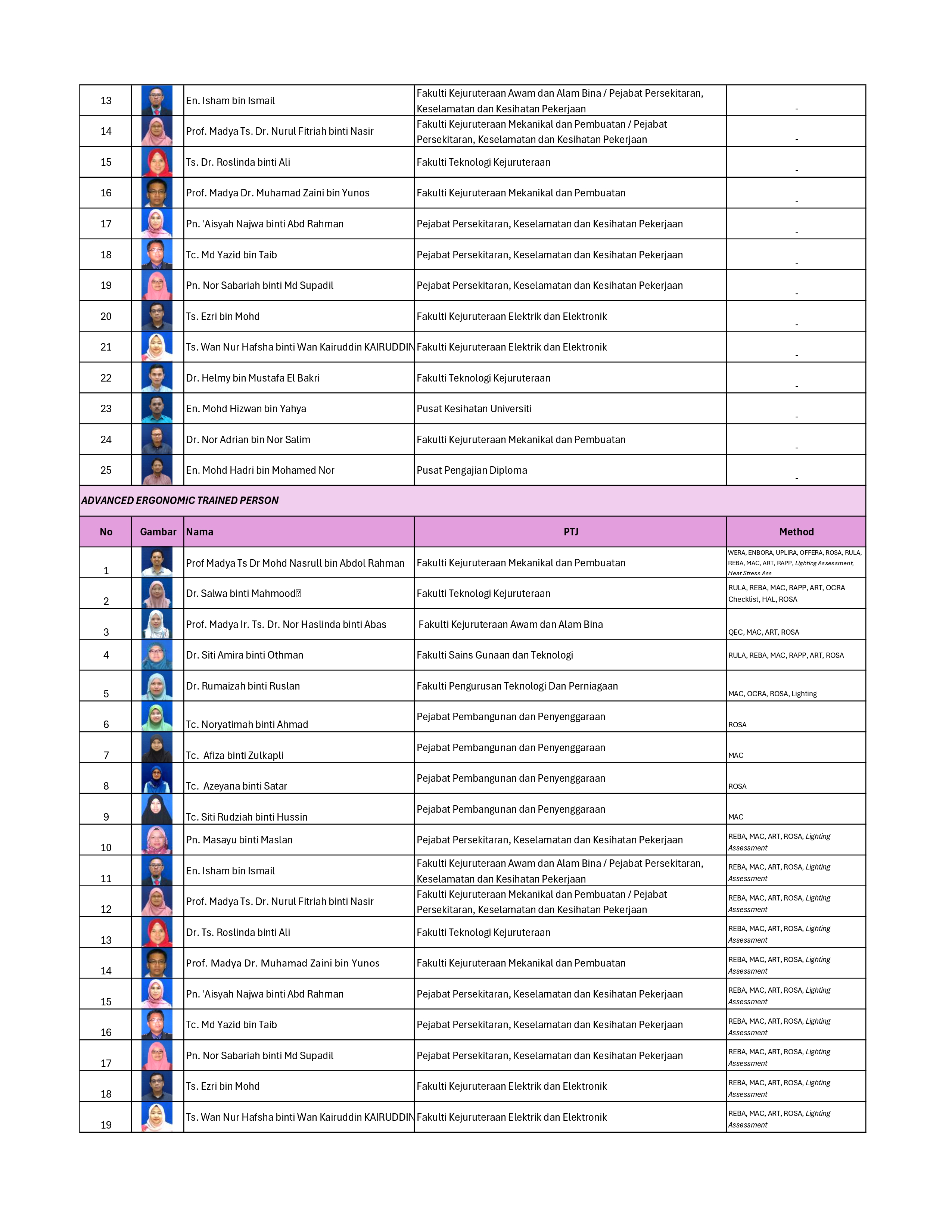
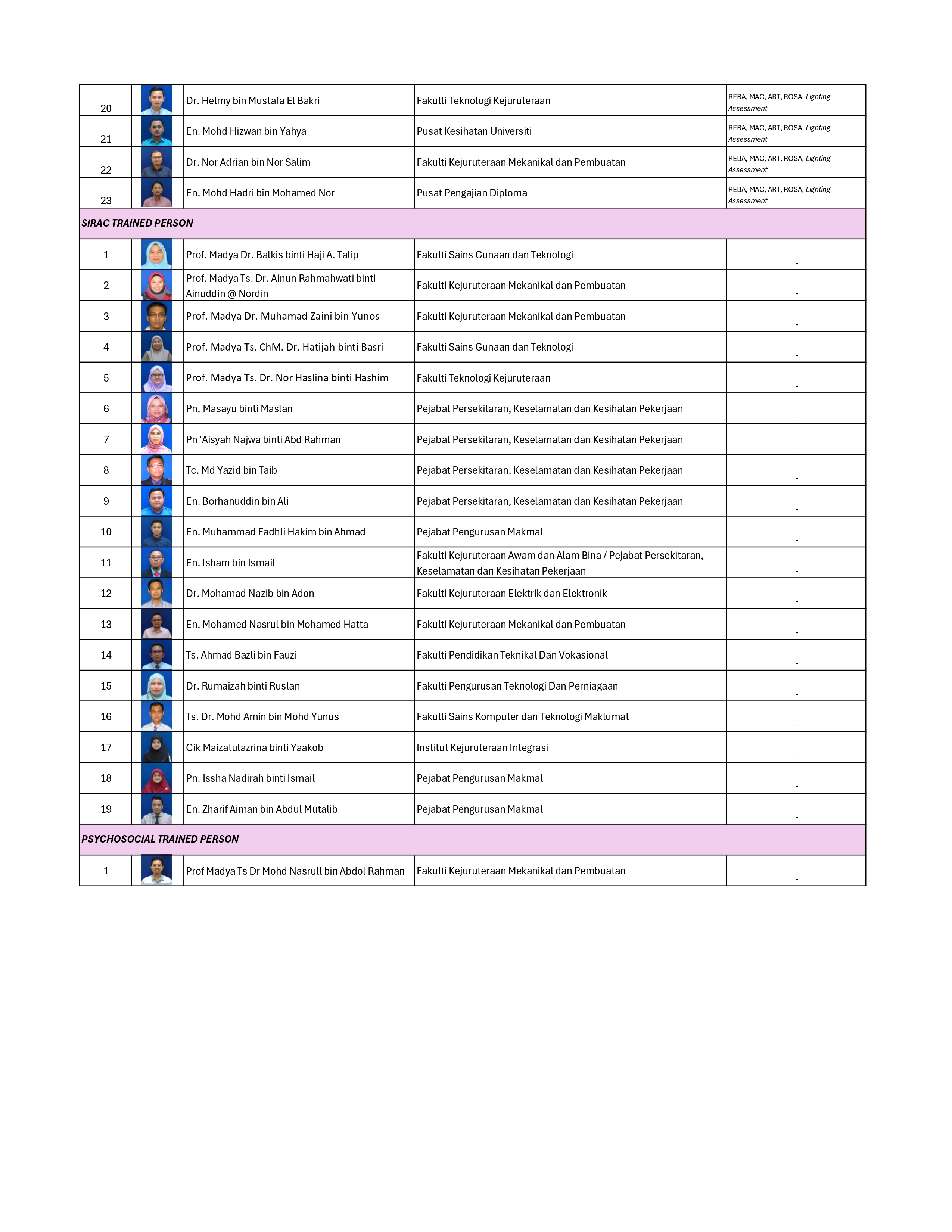
Read more …SENARAI OYK/OYT STAF UTHM
- Hits: 755




Read more …SENARAI OYK/OYT STAF UTHM

Kursus yang telah diadakan pada 5 Oktober 2017 bertempat di Bilik Latihan Seri Teratai Aras 1, Kompleks Pejabat Pendaftar. Kursus separuh hari tersebut dikendalikan oleh Prof. Madya Engr. Dr. Abdul Mutalib Bin Leman dari Fakulti Teknologi Kejuruteraan dengan dibantu oleh beberapa orang pelajar Pasca-Siswazah dibawah seliaan beliau.
Kursus ini telah berjaya menarik penyertaan seramai 60 orang peserta daripada Sembilan Pusat Tanggungjawab yang dijemput.
Objektif utama penganjuran kursus ini adalah untuk memberikan kesedaran serta penerangan kepada warga kampus, khususnya yang terlibat secara langsung dengan kerja-kerja di lokasi yang kedap udara dalam jangkamasa yang panjang, mengenai kepentingan mengambil peduli mengenai tahap pengudaraan di persekitaran lokasi pekerjaan.
Meskipun dalam masa yang agak singkat, namun penganjuran kursus ini telah dapat membuka mata para peserta kursus akan kepentingan untuk memastikan sistem pengudaraan di lokasi kerja seharian berada pada tahap yang baik dan tidak membahayakan.

Kursus tersebut telah diadakan pada 2 hingga 4 Oktober 2017 bertempat di Bilik Latihan Seri Teratai Aras 1, Kompleks Pejabat Pendaftar. Kursus telah dihadiri oleh seramai 23 orang peserta yang terdiri daripada staf Bahagian OSHE,
Safety Liaison Officer (SLO) Pusat-pusat Tanggungjawab (PTj) serta staf yang terlibat dengan pengajaran dan pembelajaran berkaitan OSH.
Berbeza dengan kursus Juruaudit Dalaman yang telah dilaksanakan sebelumnya, ianya menekankan kepada beberapa bentuk latihan dalaman serta perkongsian pengalaman audit bersama fasilitator, Manogaran Manickam, dari QEHS Lead Auditor, mempunyai pengalaman luas dalam bidang auditan di beberapa organisasi di seluruh negara.
Pendedahan berkaitan OSH Management System OHSAS 18001 & MS 1722 adalah julung kali diadakan di peringkat UTHM. Ianya merupakan salah satu usaha pihak Bahagian Persekitaran, Keselamatan & Kesihatan Pekerjaan (OSHE) untuk memastikan pematuhan terhadap Akta Keselamatan & Kesihatan Pekerjaan 1994 dapat dilakukan serta dapat difahami sepenuhnya oleh pihak yang bertanggungjawab untuk melaksanakan penguatkuasaan.

By : Dr. Siti Fatimah Zaharah Binti Mohamad Fuzi, UTHM
Introduction
Have you heard of transgenic rice resistance towards sheath blight disease, developed by the Rice and
Industrial Crop Research Centre, Malaysia? How about a delayed ripening papaya fruit, by MARDI? Or, if you have done some research, can you imagine genetically engineered bacteria that could prevent obesity in human beings (developed by researchers at Vanderbilt University, US)? What about microorganisms that could improve our daily lives?
These are some examples of the fascinating world in modern biotechnology. Researchers are using microorganisms, plants or animals to answer relevant scientific questions. Furthermore, with some tweaks to their genetic code, microorganisms can be synthetically turned into tiny workhorses that have super properties. If it is not too much to say, synthetic biology is now the new technology.
As enthralling as all of these may sound, there are also risks which come along with this kind of technology. There could be impact on the environment, economy and even human beings. It might be complicated to hold anyone accountable for the aftermath, resulting from unregulated LMOs (living modified organisms) or GMOs (genetically modified organisms) in future.
Therefore, the scientific community itself has been the first to stress the importance of working safely with LMOs/GMOs. Because of this, many regulations and guidelines exist for LMOs/GMOs. One of them is the Biosafety Act 2007.
What is Biosafety Act 2007?
Malaysia ratified the Cartagena Protocol on Biosafety in September 2003. In 2007, a Biosafety Act was approved to regulate the release, importation and contained use of LMOs and the products of such organisms. The Biosafety Act 2007 (the Act) is drafted to be in line with the National Biological Diversity Policy (1998) and the National Biotechnology Policy (2005) and covers only modern biotechnology activities.
The Act is an enabling law where most of the operational issues will be spelt out within the regulations. After series of negotiations with stakeholders, the Act entered into force on 1 December 2009. This was later followed by the entry into force of the Biosafety (Approval and Notifications) Regulations in November 2010.
Under this Act, the National Biosafety Board (NBB) was established under the Ministry of Natural Resources and Environment (NRE) which will be responsible for the implementation of the Biosafety Act and Regulations. The Genetic Modification Advisory Committee (GMAC) consisting of experts from various science-based and other relevant disciplines has also been established to provide scientific, technical and other relevant advice to the Minister of NRE and to NBB.
1Is the Biosafety Act anti-biotechnology?
Researchers within the scientific community may see biosafety procedures as a hindrance to their research activities.
Well, that is not true. The Act follows the broad scheme laid down by the Cartagena Protocol on Biosafety (CPB), as well as its parent convention, the Convention on Biological Diversity (CBD). Just like the Protocol, Malaysia recognises the twin aspects of modern biotechnology: the great potential offered by modern biotechnology and the need to protect human health and the environment from the possible adverse effects of the products of biotechnology. This is best reflected in the words of Malaysia’s former Prime Minister, Tun Dato' Sri Haji Abdullah bin Haji Ahmad Badawi, at the International Scientific Conference 2005 with the theme “Biodiversity: Science and Governance” in Paris, France,
‘….while Malaysia is aware that biotechnology holds much promise, we are also concerned that biotechnological products should not pose any threat to the environment, or to human health and safety’.
Malaysia is forging ahead in implementing legal and administrative measures on biosafety. Currently, any LMOs/GMOs intended for cultivation, contained use, field trials or consumption must be approved by the NBB, assisted by GMAC.
Why is the Biosafety Act 2007 important?
The international community has recognised the potential hazards and risks of genetic engineering. In recognition of this, the Biosafety Act 2007 establishes a process to vet all applications for the direct release of LMOs into the environment to ensure that the particular LMO is safe. If it is safe, then it is approved.
To arrive at this decision, a science-based risk assessment report provided by the applicant is reviewed by GMAC. The process is suggested by the CPB. There is no a priori (preconceived) assumption against biotechnology or the approval of the LMO. The Board has to make the decision taking into account all the necessary factors and as well as public interest. The process is also transparent.
To provide a brief overview, the 2007 Act can be divided into eight parts:
Part I –mainly concerns nomenclature and definitions used in the 2007 Act Part II –concerns the establishment of the National Biosafety Board Part III – applies to release activities and import activities involving LMOs Part IV – applies to export, contained use and import for contained use activity Part V – applies to the risk assessment and risk management reports and emergency response plan Part VI – mainly concerns the enforcement and its enforcement personnel Part VII – concerns miscellaneous issues such as confidential business information, labelling and public disclosure.
As mentioned by 2Prof. Dr Gurdial Singh Nijar in a recent NRE seminar, the Act has strengthened national decision making for the introduction of GMOs. Several other changes pertaining to GMOs and their products have come about as a result of the Act. For example, The Food Regulations 1985, under the Food Act 1983 was amended in 2010, which includes a new provision on labelling matters.
Since the Biosafety Regulations came into force in 2010, the number of applications for both notification and approval has substantially increased. The NBB has recorded a rising trend of applications since 2010 until June 2016 – from 9 in 2010 to 53 until June 2016; a five-fold increase.
Under the notification procedure under the Act, several GM crops have been created during the experimental stage in contained facilities which require notification under the Act (section 22). Some of the research work done in local research institutes and universities include: modified rice that resists the Tungro virus; papaya that has been altered to resist ring-spot virus infection and for prolonged shelf life; pineapples manipulated to resist ‘black heart’; bananas for delayed ripening; papaya for delayed ripening; chilies for virus resistance. They are also developing genetically-engineered oil palm to increase its beneficial oil content and to create nutraceuticals (vitamin A and E); However, these products are not ready to be commercialised for export yet as European consumers oppose GM products.
In the livestock and animal husbandry sectors, several animal recombinant and plant-based vaccines have been produced to assist the development of the industry. Marker-assisted breeding strategies are practiced to increase the efficiency of livestock breeding. In the treatment of industrial and agricultural wastes, the application of bioremediation techniques through bio-augmentation has been carried out. Plants (47%) and microbes (39%) were the main modern biotechnology activities under the notification act for contained use procedures from 2010 until June 2016.
This Act will ensure that the potential adverse impacts of modern biotechnology are minimised and managed in a manner that does not have a negative impact on conservation of biodiversity and human health.
And what does it mean when we say adverse impacts on conservation and biodiversity? It means that some organisms in the ecosystem could potentially harm others, due to the use of LMOs/GMOs. This in turn could lead to a lower level of biodiversity and disrupt the food web which could affect our food supply.
Conclusion
In conclusion, the national interest is best served by applying a balanced approach in promoting biosafety as well as embarking on further research that would benefit mankind. Thus, the Malaysian government is fully committed to discourse on issues of biosafety brought about by changes in which GMOs and their products are introduced, produced and ultimately consumed.
What is more challenging is to ensure that these are further enhanced by strong and continuing support from the researchers, private sector, and the rest of society. Researchers should by now realize the potential impact of GMOs and that they have the responsibility in making sure that the Biosafety Act is followed. Moreover, researchers can play a more active role in designing future awareness-raising programmes for the public.
Further reading: